Periodic Table Groups Definition

The modern periodic table is based on the modern periodic law put forward by the english physicist henry moseley, which states that “the properties of.
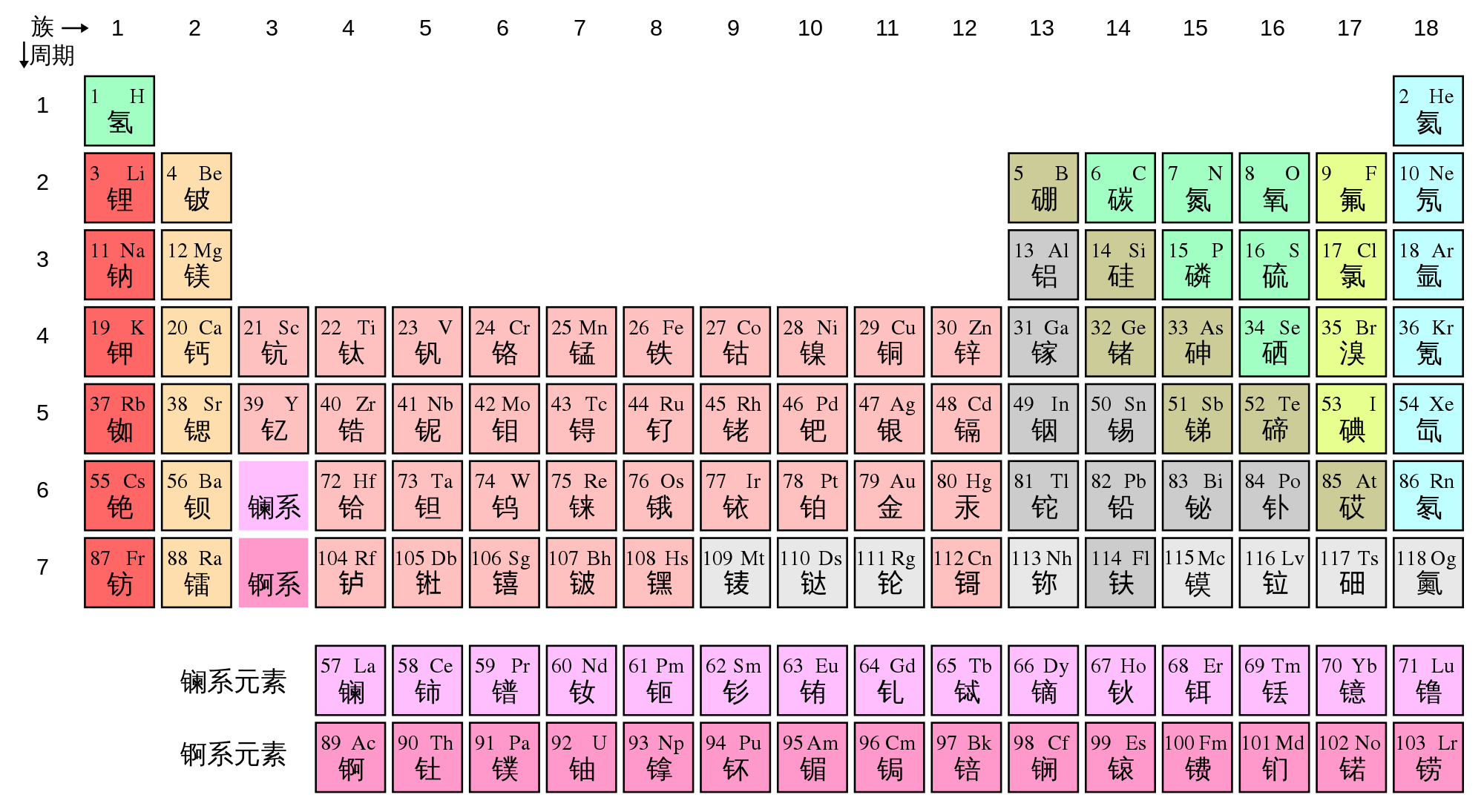
Periodic table groups definition. The figure below shows the most commonly used form of the periodic table. In the periodic table of elements, there are seven horizontal rows of elements called periods. This is what is meant by periodicity or periodic table trends.
the groups are the vertical columns. There are 18 numbered groups in the periodic table; This video will explore the representative elements, focusing on each group or family.
There are a total of 18 groups. There are 18 columns or groups and different groups have different properties. Flip through this interactive periodic table of elements.
In chemistry, a group is a column of elements in the periodic table of the chemical elements. These high definition images print and resize cleanly. Check out this free, online educational resource.
Periodic table groups are columns of elements found in the modern periodic table. There are total 18 numbered groups in the modern periodic table, however, the “f” block columns between the group 2 and 3 are not numbered. The number of each element corresponds to the number of protons in its nucleus (which is the same as the number of electrons orbiting that nucleus).
When the chemical elements are thus arranged, there is a recurring pattern called the “periodic law” in their properties, in which elements in the same column (group) have similar properties. Watch this brief video about the periodic table and element groups, from crash course. A periodic table is a tabular positioning of elements consisting of horizontal periods and vertical groups that helps in predicting the physical and chemical properties of the elements.