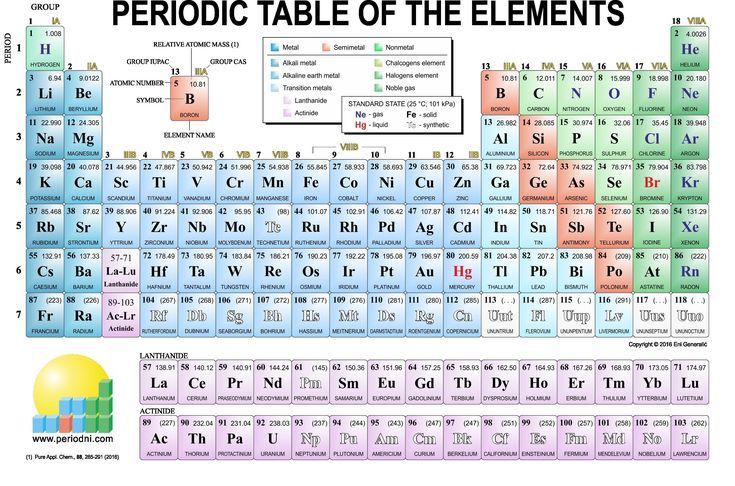
Periodic Table Groups 1 8

The elements in each group have the same number of valence electrons.
Periodic table groups 1 8. Write/draw your findings on the blank page provided. Columns of the periodic table typically mark groups or families. There are total 18 numbered groups in the modern periodic table, however, the “f” block columns between the group 2 and 3 are not numbered.
All the metals react : These are the horizontal rows that show the number of shells of electrons an atom has A group is any column on the periodic table.
Periodic table groups and families; These are labelled from 1 to 18 under current iupac numenclature. In chemistry, a group (also known as a family) is a column of elements in the periodic table of the chemical elements.there are 18 numbered groups in the periodic table;
Alkali metals, or lithium family; • he grouped elements according to their atomic mass, and as he did, he found that the groups had similar chemical properties. See about the periodic table for information on how group can be used to characterize an element.
The elements in a group have similar physical or chemical characteristics of the outermost electron shells of their atoms (i.e., the same core charge), because. They exist as single atoms. A period is a horizontal row on the periodic table.
Placed in the vertical column on the far right of the periodic table. Metals are very reactive with chemical reactivity increasing down the group. They include lithium (li), sodium (na) and potassium (k).